Self-Assessment for Depression
Esketamine Treatment for Depression
The first nasal spray medication for treatment-resistant depression
Ketamine is a medication that has been used as a surgical anesthesia around the world for more than 50 years with children, adults, and animals. About 20 years ago, ketamine’s ability to relieve depression, anxiety, and some pain disorders in less than 24 hours after administration was stumbled upon by researchers. Follow-up studies replicating the rapid results have changed researchers’ understanding of depression, revitalizing an area of medical research that had been stagnant for almost three decades.
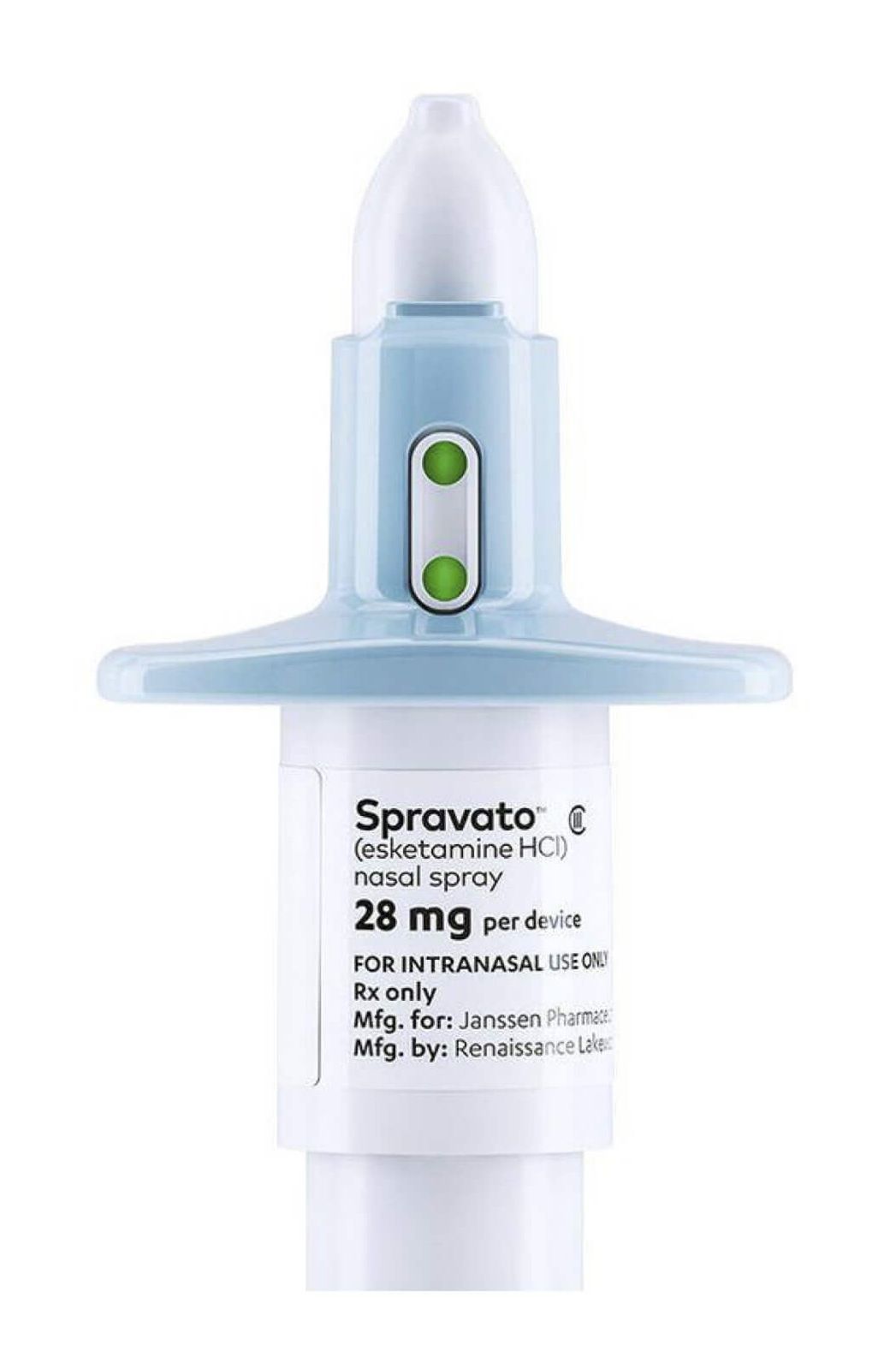
Esketamine, or Spravato, is derived from ketamine and makes powerful antidepressant relief available to be delivered intra-nasally, similar to a common nasal decongestant. Westwood Minds offers esketamine therapy in our Westwood, Los Angeles office, and this novel therapy for treatment-resistant depression can be combined with other treatment modalities, such as antidepressants, Transcranial Magnetic Stimulation (TMS), or other therapies.
What is esketamine therapy?
Esketamine, or Spravato, is the first nasal spray medication that is a treatment for depression. Combined with an antidepressant, Spravato is FDA-approved to treat depression and has been clinically shown to improve symptoms of depression over just 4 weeks.
What is an esketamine treatment like?
At our office, the patient will administer the Spravato treatment spray under the supervision of one of our healthcare providers. Afterwards, there is a two-hour observation window to monitor for side effects.
Unlike antidepressants, which need to be taken every day, Spravato is only administered a couple of times each week: Two treatments per week for the first month, then one treatment per week for the second month, and one treatment every two weeks for subsequent months.
Esketamine therapy patients generally report some sedation and disassociation effects during or shortly after the administration of the drug. Other side effects may include nausea, Esketamine therapy patients generally report some sedation and disassociation effects during or shortly after the administration of the drug. Other side effects may include nausea, dizziness, vertigo, or headache., vertigo, or headache.
Is esketamine therapy safe?
Spravato has been FDA-approved since 2019 and evaluated in both short-term and long-term trials for safety and efficacy. Because Spravato is administered in our office, patients will be monitored closely for any adverse side effects.
Self-Assessment for Depression - Contact Form
Thank you for contacting Keystone Psychiatric Services!
We will get back to you as soon as possible.
Please try again later.
Westwood Psychiatry
Phone
Hours
Monday - Friday: 9 AM-7 PM
Saturday: 9 AM-2 PM
Sunday: Closed
Now Serving
Beverly Hills
Westwood
Culver City
Los Angeles
Marina Del Ray
Santa Monica
West Hollywood
Brentwood and surrounding
Services